What is a battery?
According to the definition provided by Iran’s national standard, the battery is a source of electrical energy production through the conversion of chemical energy. Two or more cells that have an electrical connection in a circuit are called batteries.
Definition of nickel cadmium battery
Nickel-cadmium battery (in English: Nickel–cadmium battery) (English abbreviation: NiCd or NiCad) is a type of rechargeable battery that uses nickel (III) oxide and cadmium as metal as electrodes. The main use of this type of batteries is in electronic devices such as telephones or wide industries.
When was the battery invented?
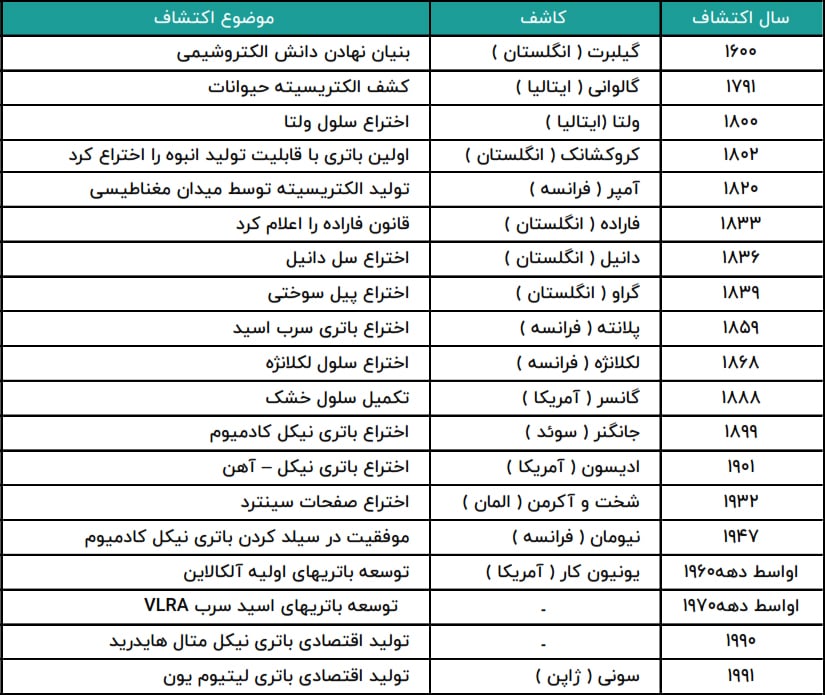
One of the most important discoveries in the last 400 years by man has been electricity, and it is surprising that electricity became beneficial to mankind from the end of the 19th century.
party battery
Recent archaeological discoveries show the use of electricity in ancient times. These discoveries show that the Iranians invented the battery around 250 BC (around 2000 BC) and probably used it to plate metals with silver.
These ancient batteries were actually clay jars inside which an iron rod surrounded by a copper cylinder was placed and its lid was sealed by bitumen, and when it was filled with vinegar as an electrolyte, a voltage of 1.1 volts was produced. DC will generate.
New age batteries
In 1800, Alexander Volta invented the first modern electric battery. Volta showed that when metals and chemicals come into contact with each other, an electric current is produced.
The next stage was the production of electricity through electrolysis, Volta realized in 1800 that a continuous current force could occur when some liquids were used as conductors to accelerate the chemical interaction between metals.
Volta also discovered that the voltage increases by collecting the Volta cells, and this discovery led to the invention of the Volta battery.
This cell consists of alternating plates of copper and zinc separated by thin cardboard plates immersed in a salt solution.
In 1802, Dr. William Cruikshank designed a type of battery that could be mass produced. Cruickshank placed square plates of copper, soldered at the ends, with equal-sized plates of zinc, alternating in a long wooden box sealed with cement. Then the metal plates were taken out from the grooves and the box was filled with electrolyte, salt water or acid water.
In 1859, French physicist Gaston Plante invented the first rechargeable batteries. The secondary batteries produced were based on lead acid, a chemical process that is still used today.
In 1899, the Swedish Jungner succeeded in inventing the nickel-cadmium battery and in 1947, Neumann succeeded in making the nickel-cadmium shielded battery. Also, research in the field of manufacturing nickel hydride batteries began in the 1970s, but due to manufacturing problems, this type of battery was practically produced commercially from the 1990s.

In 1802, Dr. William Cruikshank designed a type of battery that could be mass produced. Cruickshank placed square plates of copper, whose ends were soldered, alternately with equal-sized plates of zinc in a long wooden box sealed with cement. And she took out the metal plates from inside the grooves and filled the box with electrolyte, salt water or acid water.
In 1859, French physicist Gaston Plante invented the first rechargeable batteries. Secondary batteries produced were based on lead acid, a chemical process that is still used today.
In 1899, the Swedish Jungner succeeded in inventing the nickel-cadmium battery and in 1947, Neumann succeeded in making the nickel-cadmium shielded battery. Also, research in the field of manufacturing nickel hydride batteries began in the 1970s, but due to manufacturing problems, this type of battery was practically produced commercially from the 1990s.
Battery definitions
The definitions presented below are based on the national standard of Iran and for nickel-cadmium batteries:
- Battery:
A source of electrical energy production from chemical energy conversion
- Battery and cell:
Two or more cells that have an electrical connection in a circuit is called a battery. - Secondary cell:
A cell is a voltage generator that, after discharge, is able to return to its initial condition (charge state) through the passage of current in the opposite direction of discharge. - Port:
The release of gas due to the increase of the internal pressure of the cell or battery, which is considered in the design to prevent the explosion.
- Porous cell:
It is a cell in which, under normal working conditions, the products of electrolysis are freely dispersed in the air. - Cell with adjustment valve:
It is a cell in which the products obtained from electrolysis are stored in a sealed chamber under normal working conditions. Each cell or unit is equipped with a pressure release valve. - stationary cell:
It is a cell that is installed in a fixed place and is usually not moved from one place to another during its life.
- Page :
It is a unit that is immersed in the electrolyte alone or as a group, so that it forms the whole or part of one of the electrodes of the cell. - Separator:
It is an insulating structure that is used to separate plates with opposite polarities. - Electrolyte:
A liquid or solid substance contains mobile ions that cause ionic conduction of the phase.
- Dry cell or battery:
It refers to a ready-to-use cell or battery with non-spillable electrolyte. - Charging operation:
Passing electric current through the cell in such a way that it changes its chemical conditions and enables the cell to supply electricity to the external circuit. - Discharge:
The cell is connected to an external circuit, so that the current flows in the cell against the charge direction.
- Open circuit voltage:
The potential difference between the battery poles in the open circuit state is referred to as the open circuit voltage. - Closed circuit voltage:
It refers to the potential difference between the battery poles at the moment when the current passes through the circuit. - Rated voltage:
The voltage in the determined open circuit state of a cell.
The nominal voltage of a battery containing n series of cells is equal to the product of the nominal voltage of one cell multiplied by n.
The nominal voltage of a single nickel-cadmium cell is 1.2 volts.
- Nominal capacity:
It is the amount of electricity that is discharged from the cell at a certain rate of discharge, under certain conditions of voltage and temperature, and is expressed in terms of ampere-hours or watt-hours. The nominal capacity for a porous nickel-cadmium cell is the amount of 5C electricity declared by the manufacturer. It is in terms of ampere-hours (Ah) that a single cell can discharge at a nominal discharge with a current of 0.2 A5C and within 5 hours to reach the final voltage of 0.1 V at a temperature of 20 degrees Celsius after charging and storage, under the specified conditions. to release - Final discharge voltage:
The final voltage is the voltage that is considered as the lowest voltage limit and the end of the discharge during the discharge test and in the closed circuit mode. - Nominal effective internal resistance:
The nominal effective resistance Re declared by the manufacturer in ohms and at a temperature of 20 degrees Celsius is the value of the resistance inside the cell.
Reminder – The effective internal resistance defines an approximate linear relationship between the steady-state discharge current and the average cell terminal voltage.
- Storage time:
The duration of storage or storage in the specified conditions, until the end of that period, the battery is still able to provide the specified specifications. - Charge survival (capacity survival):
A percentage of the nominal capacity that a cell or battery delivers after being stored at a certain temperature and for a certain period of time without recharging. - Discharge depth:
It is the ratio of the capacity used during the discharge period to the nominal capacity
- Deep discharge:
Discharging 90% of cell capacity in amp hours. - leakage:
It is the unauthorized escape of electrolyte, gas or other substances from the cell or battery. - Cycle:
The sequence of charge and discharge in a secondary battery
Nickel cadmium batteries
In the following, we will discuss more about the characteristics of nickel-cadmium batteries with pocket plate technology. Nickel Cadmium battery is the best type of battery available in today’s market for working in harsh conditions.
Nickel cadmium batteries have unique features that make them widely used in applications and environments unusable for other batteries. So it’s no surprise that NiCd batteries have become the first choice for users looking for a reliable, long-life, low-maintenance system.
Hence, Tajhiz Omid Pouyesh Company by obtaining the official representative of Taihang Company (TAIHANG) China, the leading manufacturer of industrial nickel cadmium batteries; It has made it possible for this equipment to be available for employers and small and large industrial companies. You can click here for advice on Taihang batteries and to place an order
Classification of nickel-cadmium batteries based on construction
- Nickel cadmium battery with perforated pocket plate
- Nickel cadmium battery with porous sintered plate
- Nickel cadmium shielded battery
Classification of nickel-cadmium batteries based on discharge rate
- Low Discharge Rate
- Medium Discharge Rat
- High Discharge Rate
- Extra High Discharge Rate
In the classification of batteries based on discharge speed, in the first 3 rows, pocket plate technology is used and the 4th row has sintered plate technology.
Chemical process of nickel-cadmium batteries
In nickel-cadmium batteries, nickel hydroxide is used as the active material for the positive plates, and cadmium hydroxide is used for the negative plate.
The electrolyte is an aqueous solution of potassium hydroxide with small amounts of lithium hydroxide to improve the life and duty cycle at high temperatures.
The charging and discharging reaction of the nickel-cadmium battery is based on the following method:
During discharge, trivalent nickel hydroxide is reduced to divalent nickel hydroxide, and cadmium is converted to cadmium hydroxide on the negative plate.
During charging, the reaction is reversed and the cell voltage increases.
2Ni(OH)2 + Cd(OH)2
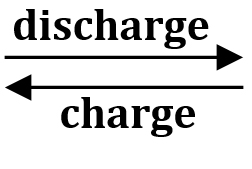
2 NiOOH + 2H2O + Cd
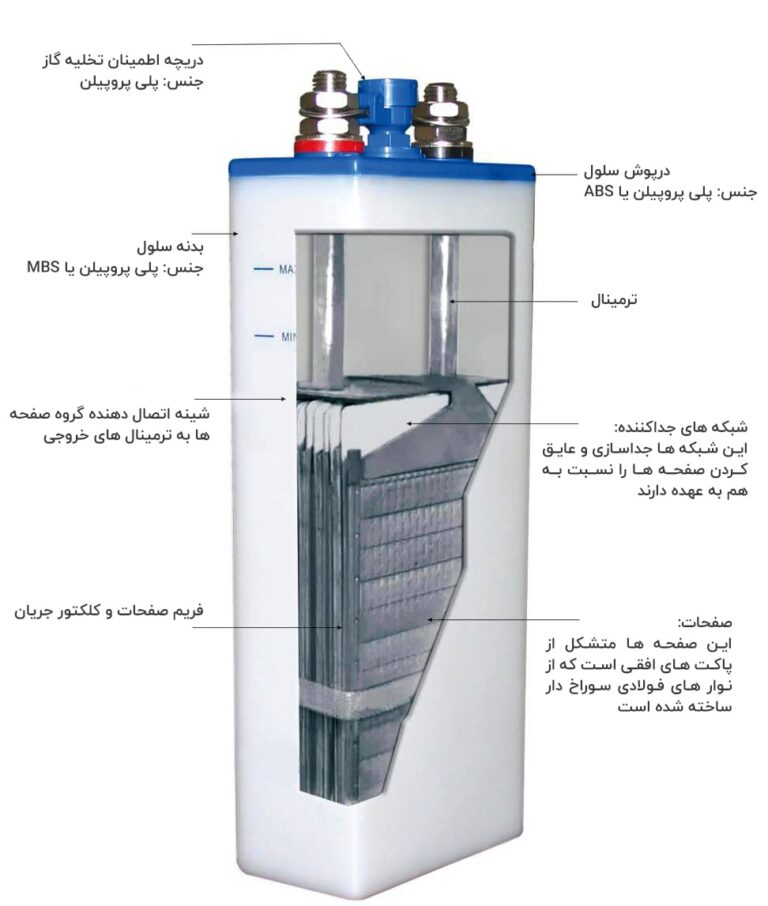
Cell structure in nickel cadmium pocket plate batteries
Positive and negative active materials are respectively perforated in steel strips and pressed in the form of a pocket that forms positive and negative electrodes. Between the positive and negative electrodes there is a separator made of non-conductive material. Electrode holders and terminals are firmly installed in a plastic case.
There is a hole on the cell door to fill the electrolyte, this hole is equipped with a plastic gas plug that can be opened whenever it is necessary to refill the electrolyte. This cap can release the gas produced inside the nickel-cadmium battery and prevent dust and pollution from entering the battery.
Applications of nickel cadmium pocket plate batteries
Nickel-cadmium pocket plate batteries are divided into the following three categories based on their function (different discharge currents):
with low discharge rate L (Low Discharge Rate)
Type L is designed for applications where the battery is required to provide a reliable source of energy with a relatively long (more than 3 hours) and uniform discharge time, and low discharges are typically required.
The maximum allowed current in the L model battery is up to A 5C 0.5 (for example, for a 100Ah battery, the maximum allowed current is up to A5C 0.5, i.e. 0.5 * 100A = 50 A).
with medium discharge rate M (Medium Discharge Rate)
Type M is designed for applications where discharge times are between 30 minutes and 5 hours, or where there is a mix of loads with high and low discharge rates.
In nickel-cadmium batteries, discharge can happen frequently or rarely, and the maximum allowable passing current is up to A5C 3.5.
with high discharge rate H (High Discharge Rate)
Type H is designed for applications where relatively high current is required with short periods and usually the duration of the period will last less than 30 minutes (generally for starting).
In this type of battery, the discharge can happen frequently or rarely, and the maximum allowable passing current is up to A5C 7.0.
Features of Ni-Cad batteries
- They have a very high lifespan and working time (they have more than 2000 charge and discharge cycles).
- High durability and endurance against electrical and mechanical stresses that threaten the UPS in the environment.
- Their rate of automatic discharge is less than other batteries.
- Their internal resistance is very small.
The superiority of nickel-cadmium batteries over other batteries
- They have high endurance.
- It is easier to maintain these types of batteries.
- The useful life of nickel cadmium battery is longer than other batteries.
- They take up very little space.
- These batteries are light and easier to transport.
Avoiding "memory effect" in nickel-cadmium batteries
To keep your Nickel Cadmium batteries in good condition, it is recommended to store them empty and in a cool, dry place. After a year or two, it’s best to charge and discharge them several times to regenerate them. The reason why it is useful to completely discharge them before charging is to deal with the mysterious phenomenon called “memory effect” that occurs exclusively in nickel-cadmium batteries.
When you regularly charge a nickel-cadmium battery after a short period of use, the battery seems to “remember” to charge more after a certain amount of charge. As a result, when you try to use more capacity beyond the normal charge level, the battery becomes lazy and fails. It’s almost like a stubborn child asking for more candy before they finish their homework.
To combat this annoying battery condition, it is recommended to regularly fully discharge your NiCd battery before discarding it and fully charge it before use.
Revival of dead nickel-cadmium batteries
Even if nickel-cadmium batteries look dead and have no charge after years of being unused, they can be revived and brought back to life by doing some “deep cycling”.
These batteries come back with depleted capacity at first, but the more you charge and discharge them, the more they start working like new batteries. The deep cycle process requires a shock from a larger battery, using multiple wires.
Buying nickel cadmium batteries
It is not difficult to buy nickel cadmium batteries with Topkwasela. For this, it is enough to have only basic information about the size of the battery you want, its capacity and voltage, and contact our experts and consultants through one of the ways of contact form with TAPCO, phone or e-mail, so that it can be properly examined. and be a guide and register the ideal order for you or your business.
