Who made the first battery?
At first, you may imagine that the first batteries made in the world are about 200 or eventually 300 years old; But this history is specific to modern batteries. It is interesting to know that the first batteries were invented by the ancient Iranians (Ashkanis) and they used it for industrial purposes thousands of years before the discovery of electricity.
Parti battery, the first man-made battery
From the beginning of the 19th century to the middle of the 20th century, the world knew Alexander Volta as the inventor of the battery, but archaeological discoveries in the 20th century showed that Iranians invented it around 200 years before Christ (2000 years before Alexander Volta). .
For the first time in 1938, Wilhelm Konig, a German archaeologist, reported the existence of Parthian piles in an article in the journal Forschungen and Fortschritte.
Wilhelm Koenig, who was in charge of the National Museum of Iraq, while exploring the area of Khaja Rabi, one of the villages near Baghdad (near Tisfon), came across a 14 cm high clay jar that had a copper cylinder inside and that cylinder also contained an iron rod. The door was closed, and investigations revealed the existence of an acidic substance, such as vinegar, in that clay container.
Before Koenig, other archaeologists had found broken pieces of these mysterious jars. However, she was the only one who realized their importance and found more of these batteries in her other discoveries. Investigations showed that the history of these batteries goes back to more than 2 thousand years ago; That is, when the Parthians ruled over Mesopotamia, which has been a part of Iran since the time of Cyrus the Great.
The Parthians were the third great kings of Iran who established a glorious empire from 248 BC to 224 AD.
The publication of Koenig’s article surprised archaeologists and the world, and researchers are still doing research on the Parthian Battery, which is also known as the Party Battery or Baghdad Battery.
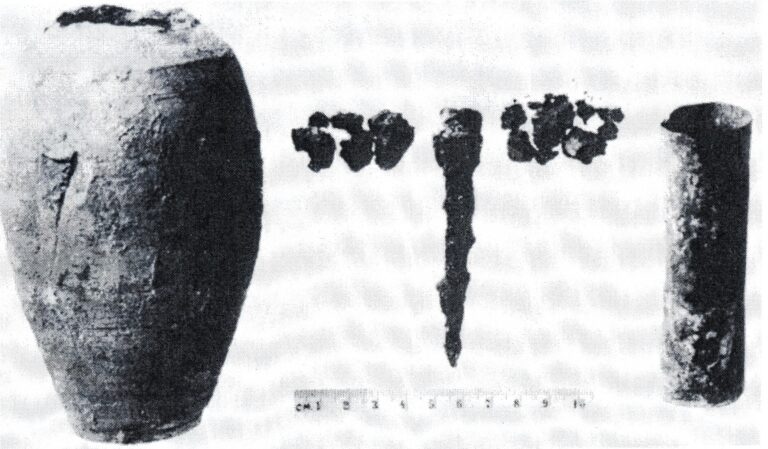
Features of Parthian battery (Party battery)
This object is actually an egg-shaped clay jar with a height of 14 cm, with a 7.5 cm long iron rod placed vertically in the middle, which acts as the negative pole of the battery, and around the iron rod, There is a copper cylinder 9.8 cm long, which is fixed in place with the help of bitumen and acts as its positive pole, and its opening is completely sealed by bitumen.
Regarding the electrolyte, it seems that salt water, copper sulfate, vinegar, lemon juice, grape wine or citric acid (which were known at that time) were used as electrolytes. Theoretically, the Ashkanian battery can produce 0.79 volts, but with the tests conducted with the help of a simulated battery and the use of various electrolyte solutions, it was proven that these batteries can provide a voltage equal to 0.5 volts DC and a current of several milliamps. .
Applications of Ashkanian battery (battery party)
There is no doubt that Iranian batteries produce electricity, because many students in various universities have built samples and produced electricity. But what did the Parthians use this device for?
The discovery of this battery raised various assumptions. Production of electric current (hypothesis of power source), gold and silver plating on other metals (hypothesis of metal plating) and use in the treatment of diseases with electric shock (hypothesis of medical use) were all hypotheses that were proposed in this field. But with the discovery of samples of plated dishes, bracelets and ornaments around the place where these batteries were discovered, the hypothesis of plating gold and silver on other metals seems certain.
After the Parthians, the use of batteries was forgotten because there was no science in this field.


New age batteries
After the Parthians; With the efforts of Galvani and Volta (late 18th century), batteries of the new era were invented and caused a tremendous transformation in the scientific communities and the electricity industry.
Luigi Galvani
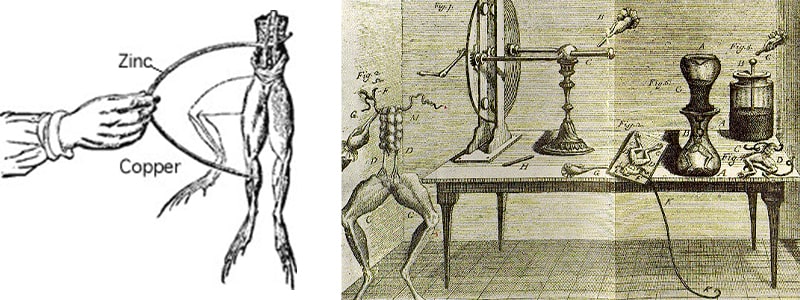
In 1791, Luigi Galvani, a famous Italian scientist, physician and physicist of the 18th century (1737-1798 AD), discovered that if the muscles of a dead frog are subjected to a spark or electric shock, they will expand and stretch strongly, thus almost He discovered bioelectricity by accident. She also discovered that muscles and nerve cells produce electricity.
Luigi Galvani was one of the pioneers of converting chemical energy into electrical energy.
Alexander Volta
In 1800, Alexander Volta invented the first modern electric battery. Volta showed that when metals and chemicals come into contact with each other, an electric current is produced.
The next step was to produce electricity through electrolysis. Volta discovered in 1800, using a copper coin, a silver coin and a silk cloth immersed in salt water, that a continuous current force is possible when some liquids are used as conductors to accelerate the reaction. Chemically, happen between metals.
Volta also discovered that the voltage increases by collecting Volta cells, and this discovery led to the invention of the Volta battery. This battery consisted of alternating plates of copper and zinc, between which there were thin cardboard plates immersed in a salt solution.
William Cruikshank
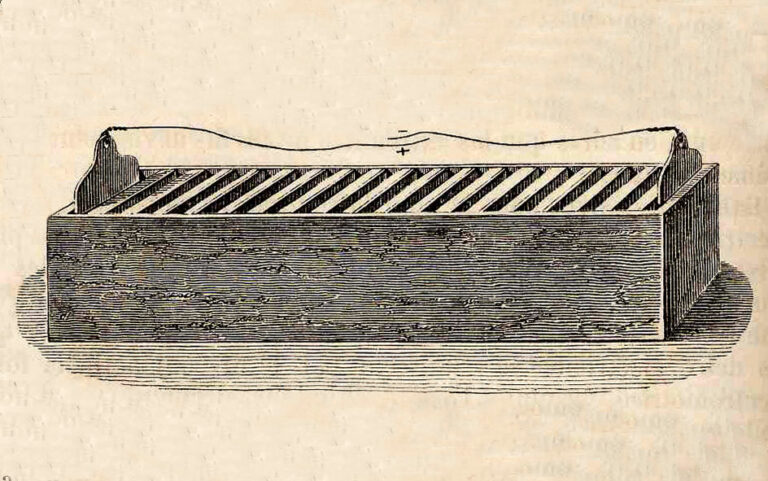
In 1802, Dr. William Cruikshank designed a type of battery that could be mass produced. Cruickshank placed square plates of copper, soldered together end-to-end, with equal-sized plates of zinc alternately in a long wooden box sealed with cement, and removed the metal plates from the leading and trailing grooves. and filled the box with electrolyte. This was the first type of battery that could be mass produced.
Types of batteries
Daniel's cell
This cell was invented in 1836 by John Frederic Daniell, an English chemist.
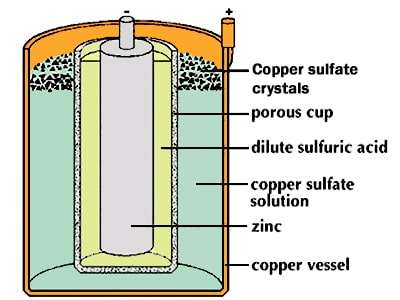
In this cell, like the Volta battery, zinc metal acted as the anode and copper as the cathode, with the difference that Daniel used two separate electrolytes to increase the battery life and solve the hydrogen bubble problem. In this way, the zinc metal was placed in the zinc sulfate electrolyte or dilute sulfuric acid, and the copper metal was in contact with the copper sulfate electrolyte (the outer shell of the battery was made of copper).
Daniel Peel was used for a long time in the European telegraph industry.

Lead acid battery
In 1859, the French physicist Gaston Plante invented the first rechargeable batteries based on the lead-acid system. After more than 170 years, this invention is still one of the most popular types of batteries. The initial model of this type of battery consisted of two spiral rolls of pure lead and lead oxide, which were separated by linen cloth and placed in sulfuric acid electrolyte.
A new model of lead-acid battery was developed in 1881 by Camille Alphonse Faure, who was a French engineer. In this model, in order to overcome the limitation of chemical reaction and make more efficient electrodes, Fauré placed thin lead paste on metal plates, which allowed these porous electrodes to have more contact with the electrolyte.


Leclange cell
This primary (non-rechargeable) cell was invented by the French Georges Lionel Leclanché in 1866. The structure of this cell includes a carbon rod surrounded by manganese dioxide powder as cathode, a zinc metal rod as anode and ammonium chloride electrolyte.
Using this electrolyte instead of acid made this cell lighter and safer. Due to its cheap price and lightness, this type of battery was used in electrical appliances, especially flashlights and portable equipment, about 100 years later, with a slight change in its construction. Today we know this battery as zinc-carbon battery.

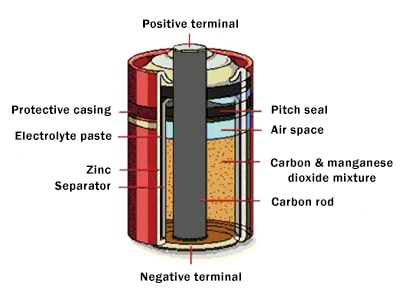
Dry cell (structural completion of zinc carbon cells)
Leclange’s cell was very heavy and broke easily. The initial idea of encapsulating batteries was considered since 1881, but the first dry cells were made by Carl Gassner, a famous German chemist, in 1887.
In these cells, the carbon negative electrode and other elements were placed in a body made of “zinc”, which also acted as a positive pole, the electrolyte was absorbed by a porous material, and the cell was sealed at the top. Also, zinc chloride was added to the electrolyte to prevent zinc corrosion during cell inactivity.
The main feature of these cells was their easy carrying and no electrolyte spillage, which was introduced as the prototype of the large dry battery industry and is still being produced without significant changes in its structure.
Nickel cadmium cell
Swedish inventor and engineer Ernst Waldemar Jungner invented the first nickel-cadmium batteries in 1899. In this battery, nickel hydroxide acts as the positive pole, cadmium hydroxide acts as the negative pole, and potassium hydroxide acts as the electrolyte.
Nickel cadmium battery was the first battery using alkaline electrolyte. At that time, this battery was much more powerful and had a higher energy density than lead-acid batteries, but it was more expensive.
Nickel cadmium battery has the ability of slow to fast charging or pulsed to uniform charging and works as a silent and powerful worker in very harsh conditions with minimal problems.
In fact, the nickel cadmium battery is the only battery among the existing batteries that can perform properly in very difficult working conditions. The rest of the battery types prefer shallow discharge and medium loads.
Nickel cadmium battery is the most economical battery in terms of its performance.
If you need to read more about nickel-cadmium batteries, go here.


Iron nickel cell
The invention of iron-nickel cells was registered in 1905 under the name of Thomas Edison, but actually the inventor of this cell was none other than the Swedish Ernst Jungner. Although Jungner invented this battery in the beginning, due to the low energy density and excessive production of hydrogen gas, she did not pay much attention to it, so Edison, by modifying and developing it, registered this battery under her name.
The main capability of this battery was in cases where the cells were charged and discharged daily, and Edison replaced lead-acid batteries in electric cars, which at the time comprised about 28% of all cars in the United States.
In this cell, nickel hydroxide is used as the cathode, iron is used as the anode, and its electrolyte is potassium hydroxide, and its nominal voltage is 1.2 V like nickel cadmium cells.
In 2011, an American researcher succeeded in reviving an 85-year-old nickel-iron battery and ready for use, which shows the exceptional ability and life of nickel-based batteries.
In the following decades of the 20th century, with the explosion of technology, the existence of two world wars, the first and second world wars, the need for better efficiency of energy storage resources in weapons, and the boundless human desire for progress and excellence, this process suddenly gained increasing speed; So that in the 20th century, in addition to the invention of cells with new technology, the development and completion of previous inventions as well as their commercialization, it increased the efficiency of batteries so much that in many cases it can even be considered as important as their invention.
Now in the 21st century, with the arrival of new and nano technologies, it is expected that the batteries of the next generation, in terms of efficiency, will behave in a way that challenges all our mental fields regarding physics.
Some important battery inventions and events in the 20th century
- 1932: Nickel-cadmium cells with sintered technology (Sintered Ni-Cd Battery)
In this year, two German engineers, Schlecht and Ackermann, invented the nickel-cadmium cell with sintered technology, which has a great ability to discharge with very high currents in short times. - Early 1940s: Silver Zinc Cells
During the early years of the 1940s, the French professor Henri Andre managed to improve and economically develop this type of batteries by changing their separator. The extraordinary ability of silver-zinc batteries is their extraordinary energy density. - 1947: Spanstrong Sealed Ni-Cd Battery
This year, the French Neumann succeeded in sealing nickel-cadmium batteries, which are currently used for various electronic equipment. - 1949: Alkaline manganese battery
In 1949, Lew Urry modified small alkaline batteries in the laboratory of Eveready Company in Parma. The performance of these cells was 5 to 8 times higher than that of carbon-zinc cells. These cells are still used in many small electronic devices, from high-tech measuring devices to toys. - 1950: Zinc-mercuric oxide alkaline battery
This battery was invented by Ruben, who was an independent inventor. Mercury compounds are no longer used due to environmental problems. - 1960: Button batteries
In this year, miniature cells called button cells were made with silver oxide technology for watches. - 1960s: strong development of primary alkaline batteries (non-rechargeable) (Primary Alkaline Batteries)
The American Union Carbide company modified the structure of these types of batteries during the 1960s. - Late 1960s: production of metal hydride (MH) cells
In these years, scientists realized that some metal compounds such as SmCo5 and LaNi5 have the ability to absorb and expel a large amount of hydrogen, which can be used as one of the poles of energy storage sources. - Early 1970s: Invention of non-rechargeable lithium batteries
In the early 1970s, different companies succeeded in producing different types of lithium-based non-rechargeable batteries. - 1970s: Development of Valve Regulated Lead Acid Battery (VRLA)
The 1970s are considered as the golden years of the development of this type of battery, in which new technologies with various capabilities were used to develop the lead-acid battery. - 1980s: Invention of rechargeable lithium batteries
In these years, rechargeable lithium batteries were made, but due to safety issues and the inherent instability of lithium, especially during charging, they all failed, which made researchers turn to lithium-ion cells.
Although lithium-ion cells have a lower energy density than lithium metal, they were used as an alternative due to greater safety. Lithium ion cells are known as cells with the ability to improve. - 1999 : economic production of nickel metal hydride (Ni-MH) cells by Japan’s SANYO company
- 1991: Economic production of lithium ion cells (Li Ion) by SONY Japan
- 1994:Economic production of lithium ion polymer cells (Li Ion Polymer) by American Bellcore company
- 1996: Economic production of lithium ion cells with manganese cathode (Li Ion Manganese) by Moli Energy of Canada
- 1996: Identification of lithium phosphate cells (Li-phosphate (LiFePO4)) by the University of Texas, USA
- 2002: Development of lithium phosphate cells using nanotechnology by the universities of Montreal, Quebec, MIT and others
Next generation batteries
With the advancement of new and nano technology, it is certain that in the near future, cells with different and strange capabilities will be produced that fully meet current needs and do not have the problems of current batteries.
Some of their types are now in the initial stages of research, which we mention:
- Cells that can be printed on paper could be a new avenue for batteries for portable electronic devices.
- Fuel cells are used as portable energy sources.
- The design and construction of a battery by the University of Missouri in the United States, which uses the decay of radioactive isotopes to produce electrical energy, and its dimensions are the size of a coin. This nuclear battery can produce a million times more electrical energy than conventional chemical batteries.
- Engineers of Japan’s NEC company succeeded in producing a flexible battery that is less than one millimeter thick and charges within 30 seconds. This battery is used in cards such as RFID smart cards and can provide sufficient power for these cards for several weeks without needing to be recharged. Some kind of organic material is used in this battery. To make this battery, a kind of plastic made of organic radical material is made in the form of a thin layer and it is used as an electric pole. The voltage of this thin battery is 7.3V, like lithium batteries. This battery has a lot of flexibility and can be bent by hand.
